
|
What's
Electricity?
|
|
|
Charge Is Fundamental
|
|
Electric charge is a fundamental property of some
elementary particles of matter, for example electrons and
quarks. Electric charge can have a polarity of either
negative or positive.
An electron is an elementary subatomic particle having one negative elementary charge
(symbol −e).
A proton is a composite subatomic particle
comprising three electrically charged
elementary particles called quarks. A quark has a
fractional charge of either -1/3 or +2/3. The three quarks in a
proton combine to produce one net positive charge (+e).
The mathematical symbol for charge is q
or Q from the 18th century phrase "quantity of electricity".
The standard unit of electric charge is the coulomb, abbreviated C
(capital C because Coulomb was a person).
Around 1910, Robert Millikan became the first person to measure the
elementary
charge, a fundamental physical constant. His value was extremely
close to today's definition :
e ≡ 1.602 176 634 x 10 -19 C
Inversely, 1 C ≅ 6¼ billion billion electrons
An alkaline AA battery delivers about 5,000 C of charge during its
useful life. An average bolt of lightning delivers just 15 C
but it does so in just 30 microseconds!
The Atom
Surrounding every electric charge is a space (a
field) acting to move the charge toward opposite-polarity charges
and away from like-polarity charges.
Electrons, being lightweight and energetic, are electrically attracted
toward the more massive and
more stationary protons.
Meanwhile, protons clump together with other protons, and also with
neutrons (particles composed of three quarks totaling zero
charge). These protons and neutrons are held together by the strong nuclear force,
which is much stronger than the electric force.
The positive clump of particles (called a nucleus) and
all its attracted electrons is called an atom.
The attracted electrons repel one another, forming a negative cloud
around the nucleus.
The diameter of a nucleus is between 1.6 and 15 femtometers,
abbreviated f m. A femtometer is also called a fermi, in
honor of nuclear physicist Enrico Fermi.
1 f m (or fermi) = 1 quadrillionth (10 -15 ) of a meter
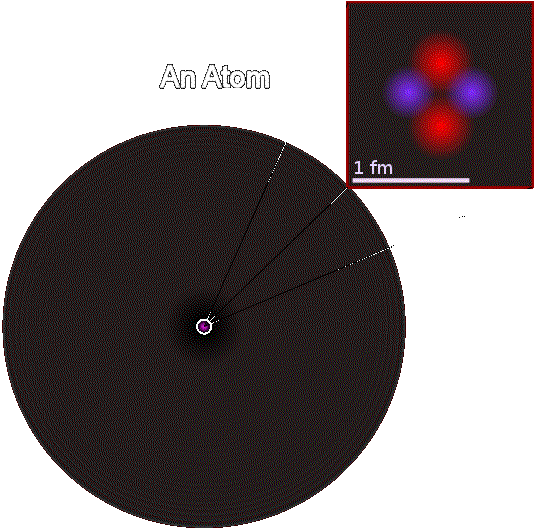
The Atomic Nucleus, Surrounded by a Cloud of Electrons
|
|
Why Don't Electrons
Stick To The Nucleus?
|
|
In 1923, Louis de Broglie, a French physics graduate student, presented
his findings attributing wavelengths to electrons.
It was already known that light waves could behave as particles called photons and de Broglie reasoned that nature was symmetric.
Why shouldn't particles behave as waves?
In 1929, after the wave nature of electrons
was demonstrated experimentally, the Nobel Prize for Physics was
conferred on de Broglie for his discovery.
A particle's de Broglie wavelength (symbol lambda λ)
is inversely proportional to its momentum :
|
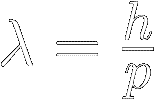
|
[1]
|
|
Where:
λ = the particle's wavelength, in meters
p = the particle's momentum (mass × velocity), in kg⋅m ⁄ s
ℎ = Planck's constant ≡ 6.626 070 15 × 10 -34 kg⋅m2 ⁄ s
De Broglie wavelengths are usually provided in nanometer (nm) or Angstrom
(Å) subunits :
1 nm = 10 -9 m
1 Å = 10 -10 m
NOTE : Equation [1] also converts a light's wavelength to its photon momentum. Although
photons
have no mass, they have momentum thanks to quantum mechanics.
An electron traveling at a reasonable speed has a wavelength of about
0.01 nm, thousands of times longer than the 1.6 to 15 f m diameter of an atomic nucleus.
Because it's so spread out in comparison, an electron can't even exist closer than a
couple of electron wavelengths from a nucleus, much less stick to one.
The Elements
Hydrogen, the lightest atom, has one proton and one electron.
Heavier
atoms have over a hundred of each. Each atomic size is one element
in the periodic table of chemical elements.
Atoms, themselves, can bond together by sharing electrons, forming
larger molecules, compounds, and
other fancy stuff.

Tennis balls bounce, buildings stand, and aspirin thins the blood,
all thanks to electric charge.
|
|
Current — Moving Charge
|
|
In the periodic table, the metal elements bond into structures
wherein many electrons
are
delocalized. In other words, they're free to move
about, not being tied to any one atom or chemical bond.
The metallic structure resembles a fixed lattice of positive ions (atoms
lacking an electron) sitting in a ‘sea’ of mobile electrons.
These mobile charges can flow en masse through the lattice much like
water flows through a sieve.
The rate of flow of these
charges is called electric
current, symbol ‘I’
from the French phrase "intensité de courant".
So, current (I) is the amount
of charge (q)
passing a certain point in space per unit of time (t)
:
|
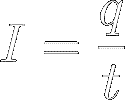
|
[2]
|
|
The unit of electric current is
the ampere or amp, abbreviated A [capital
‘A’ because French mathematician and physicist
André-Marie Ampère (1775-1836) is considered to be the dad of
electrodynamics].
▶ Although we've defined current in terms of charge, it's interesting
to note that current, not charge, is the base electrical unit in the
International System of Units (SI). There are only 7 base units
in the SI: length (meter), time (second), mass (kilogram),
electric current (ampere), temperature (kelvin), amount of substance
(mole), and luminous intensity (candela).
The SI defines the ampere as
"that constant current which, if maintained in two
straight parallel conductors of infinite length, of negligible circular
cross-section, and placed one meter apart in a vacuum, would
produce between these conductors a force [electromagnetic] equal to 2 × 10−7
newtons per meter of length".
A coulomb is then "the amount of [electromagnetic] power conveyed in
one second by a current of one ampere". In other words,
charge is defined in terms of current, as follows :
Q = It
Materials that support electric current, such as copper,
tin, nickel, silver, and gold, are
called conductors.
Materials that don't support electric current, such as wood, rubber,
ceramics, plastic, and glass are called insulators.
|
|
Voltage — Separated Charge
|
|
Potential energy arises whenever opposite electric
charges are separated, just as it does when a stone is lifted off the
earth. The energy rise comes from the energy spent in separating the
charge or lifting the stone.
The unit of energy (symbol E) is the joule
(J) in honor of James Prescott Joule, a 19th century English physicist,
mathematician and brewer who related heat to mechanical energy, laying the foundation for the law of
energy conservation.
Electrical voltage (symbol V)
is defined as the potential energy of separated charge, per unit of charge (q) :
|
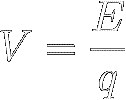
|
[3]
|
|
The unit of measurement for voltage is the volt (V)
in honor of Alessandro Volta, an Italian physicist and chemist who
invented the primary battery in 1799.
One volt
is one joule of energy per coulomb of charge.
Voltage is sometimes called an electromotive force (emf )
because voltage can change an object's motion by transferring energy to it.
But, despite its name, emf is not a force but rather a potential energy.
|
|
Power
|
|
The transfer of energy (i.e., work) can be accomplished quickly or slowly but
doing it quickly takes more power.
For example, it takes more power
to run up a hill than to walk up even though both ways give you
the same amount of gravitational potential.
Power (symbol P) is thus defined as
energy transfer per time. In other words, It's the quickness of energy
transfer :
|
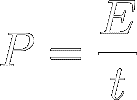
|
[4]
|
|
The unit of measurement for power is the watt, abbreviated W
(capital W because Watt was a person). 1 W is the power needed to
transfer 1 joule of energy
in 1 second.
Equation [4] can also be written :
E = P × t
In fact, your electric service is ordinarily billed in units of
kilowatt-hours (P × t), not joules.
One kilowatt-hour (kWh) is
1,000 watts of power for 1 hour (3,600 seconds), making the total
transfer of energy equal to 3.6 megajoules :
1 kW h = 1,000 W x 3,600 s = 3,600,000 J = 3.6 M J of energy

An alkaline AA battery delivers 9 kilojoules (k J) of energy in its
useful life. An average bolt of lighting delivers 1,000,000 k J in
just 30 microseconds!
Power = Volts × Amps
Take a look at the following algebraic identity, where the two q' s cancel each other out :
|
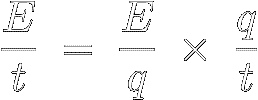
|
[5]
|
|
Since the above three ratios are the definitions of
P, V,
and I ( see equations
[4],
[3], and
[2] ),
we see that the power P of
an electric current I flowing across a
voltage V is :
|

|
[6]
|
|
One amp of current across a one volt energy difference equals one watt
of power.
|
|
Resistance
|
|
In the ordinary world, there are no perfect conductors of electric current. Even in
metals,
electrons collide with ions, losing energy in
the form of heat.
This friction-like impedance to electric current is called resistance.
In electronics, resistance can be used to limit currents and to
establish
potential differences. Components called resistors
are engineered to provide precise amounts of resistance.
Ohm's Law
Experiments show that the ratio of the voltage V across a
certain
resistor to the current I flowing through it is a constant.
This constant
ratio is defined to be the resistor's resistance (symbol R) :
|
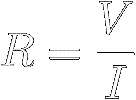
|
[7]
|
|
The unit of resistance is the ohm, abbreviated Ω
(capital Omega because Ohm was a person). A 1 Ω resistor across 1 V
of voltage will pass 1 A of current.
Equation [7] is called Ohm's Law. Multiply both sides of
Ohm's Law by I to find that
the voltage across a resistor equals the current through the resistor
multiplied by
the resistance :
|
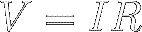
|
[8]
|
|
Divide both sides of equation [8] by R to
find that the current through a resistor equals the applied voltage divided by
the resistance :
|
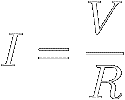
|
[9]
|
|
A trick to help remember the three permutations of Ohm's Law (equations [7], [8] and [9])
is to substitute
Vulture, Rabbit, and Indian for V, R, and I.
The
Vulture sees the Indian beside the Rabbit. The Rabbit sees the Vulture
over the Indian, and the Indian sees the Vulture over the Rabbit.
Power Dissipation
A resistor must be able to dissipate all the heat generated by the
internal electron-ion collisions; otherwise, it'll overheat and burn
out. Therefore, each resistor has a power rating.
In equation [6] ( P = V × I ) ,
we can use Ohm's Law to replace V with
IR and thus find out how much power a
certain resistance R must shed
when a current I flows through it :
|
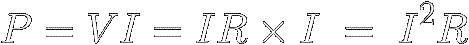
|
[10]
|
|
Or we can use Ohm's Law to replace I with V/R to find
out how much power the resistance R
must shed when a
voltage V is applied across it :
|
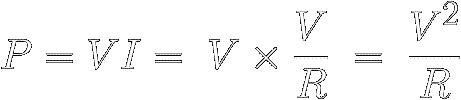
|
[11]
|
|
Resistor Construction
Like garden hoses, conductors that are long and thin offer more
resistance to current than do ones that are short and fat.
|

One way to manufacture a resistor is to coil up a long, thin piece of wire.
Wirewound resistors can be precise and also handle large currents.
|
|
Other resistors are constructed from a material, such as carbon, that falls
in between a conductor and
an insulator. Carbon has relatively few
delocalized electrons.

These vintage, carbon composition resistors are composed of tiny carbon particles bound with clay.
|
|
Many modern resistors are made from laser-cut, helical tracks of carbon
or metal film.
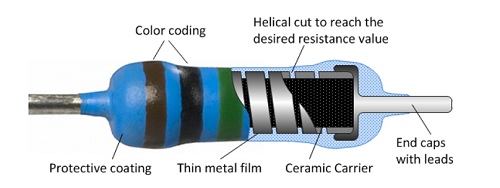
|

|
|