|
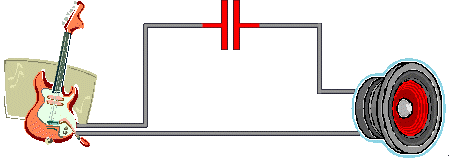
A common "parallel-plate" capacitor is made up of two sheets of conducting foil
(called plates) and a non-conducting film sandwiched in between.
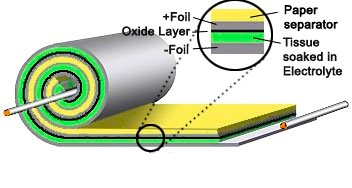
To save space, the sandwich is often rolled up like a jelly roll.
Each foil sheet has an attached lead so the capacitor can be wired
into an electrical circuit. (See illustration, below.)
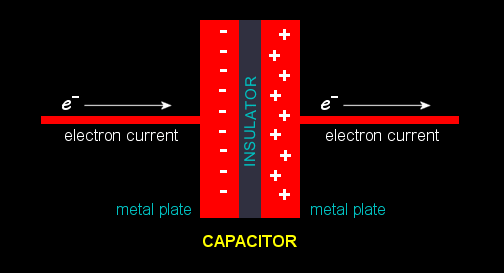
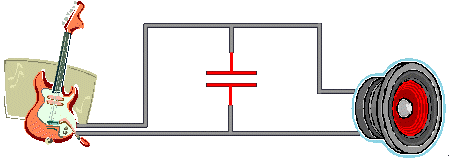
When a capacitor is in the path of an
electric current,
electrons accumulate on the "upstream" plate, giving it a negative
charge. Since like charges repel, electrons are driven off the
nearby plate, leaving behind positive ions
(atoms missing one or more electrons).
Electric
charge is now trapped in the capacitor. The electrons and the
ions attract each other but they can't travel across the insulator.
Their potential energy is stored in the surrounding electric
field,
registering as a
voltage
across the capacitor's plates.
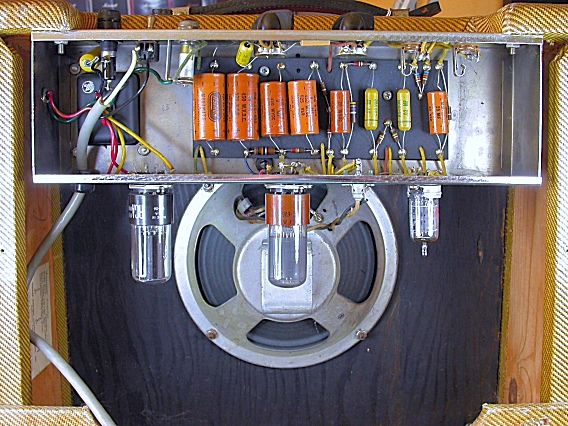
Electrolytic Capacitors
An "electrolytic" capacitor comprises two very thin foil
plates immersed in an "electrolyte" [a solution that
contains both positive and negative ions (called cations and anions)].
In the capacitor, these dissolved ions function as charge carriers.

The Layers of an Electrolytic Capaciator

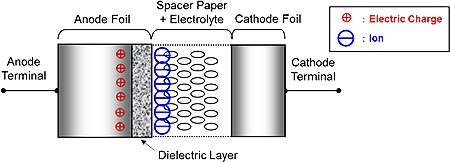
After an electrolytic capacitor is manufactured, the manufacturer
applies a DC voltage across its
foils (the electrodes) with the positive side of the voltage connected
to the "anode" foil and the negative side to
the "cathode" foil.
This voltage pushes electric charge from one electrode to the other,
through the electrolyte. Electrolysis, an electrochemical reaction,
causes a tiny oxide coating to form on the anode foil.
After its formation, the oxide coating serves as the insulator (the
"dielectric") between
the capacitor plates. To maintain the oxide coating, the capacitor must
always operate with a net DC voltage across it, with the same polarity
as the forming voltage. The capacitor is
"polarized", with a plus and a minus terminal.

Minus Terminal (Cathode) Markings on
Electrolytic Capacitors

When the capacitor is in an electric circuit, dissolved positive ions (cations)
are attracted to its more negative foil (its cathode),
and dissolved negative ions (anions) are attracted to its more positive foil
(its anode). But charge flow is dammed up by the
anode's oxide coating – the potential energy
is stored in the surrounding electric
field.

Oxide Layer Blocks Anions from Reaching the Anode

If the capacitor is accidentally connected backwards, the anode's oxide
coating is destroyed. Oxide starts to form on the cathode but, meanwhile,
charge flows unhampered through the capacitor.
This electric current heats up the
electrolyte, which can boil. The capacitor may puff up
and eventually explode. Or the electrolyte
may leak out and degrade the capacitor.
|