
|
What's
Electricity?
|
|
|
Electric Charge |
|
Electric charge is a fundamental property
of certain subatomic, elementary particles of matter. The amount of
charge determines the strength of the particle's coupling to
electromagnetic (EM) fields.
Likewise, the amount of mass determines the strength of
a particle's coupling to
gravitational fields.
Every particle with a mass is the source of a gravitational field and every particle with
a charge is the source of an EM field.
However, unlike mass, charge is polar—having either positive
or negative values. So, while the gravitational force is always attractive,
EM forces can be either attractive or repulsive.
Electrons and Protons
Two of the elementary particles that possess electric charge are electrons and
quarks.
The amount of charge held by an electron is called one negative elementary
charge ( symbol −e) .
This amount of charge is a fundamental physical constant.
The proton is a composite particle
containing 3 fractionally-charged
elementary particles called quarks. Two of
the quarks have a charge of +2⁄3 e and one has
a charge of −1⁄3 e.
So, all combined, the 3 quarks give the proton one positive elementary charge ( symbol +e
or just e).
● The math symbol
for electric charge is ‘q’
or ‘Q’, from the 18th century phrase
“quantity of electricity”.
● The unit of electric charge is the coulomb,
abbreviated ‘ C ’ — capital C because Coulomb was a person.
► Charles-Augustin de Coulomb (1736-1806) –
a French officer, engineer and physicist – discovered that the
electrostatic force between two charges is proportional to the product of the
two charges and inversely proportional to the square of the distance between them
( see Coulomb's Law ).
How Many Electrons
are in a Coulomb?
Around 1910, Robert Millikan became the first person to experimentally
obtain a value, in coulombs, for the tiny elementary charge of the electron.
Millikan's value was extremely
close to today's International System ( SI ) definition :
1 e ≡ 1.602 176 634 × 10 -19 C
By taking the inverse of the above number of coulombs/electron, we get the following result
for the number electrons/coulomb :
1 C = 6.241 509 074 460 × 1018 e
≅ 6¼ billion billion electrons
An alkaline AA battery delivers about 5,000 C of charge during its
useful life.
An average bolt of lightning delivers just 15 C
but it does so in a mere 30 microseconds!
The Atom
Recall that every charged particle is surrounded by a space ( or
field ) that's electromagnetic. ‘EM’ fields
interact,
forcing charges away from same polarity
charges and toward
opposite polarity charges.
Electrons, being lightweight, energetic and negatively charged, are
forced toward protons, which are positively charged, heavier and less mobile.
Meanwhile, protons clump together with other protons and also with
neutrons—particles made up of 3 quarks
having a sum total of zero charge.
These clumps are held together by the
strong nuclear force, which is much
stronger than the EM force.
An atom consists of one positive
clump called a
nucleus surrounded by a
cloud of attracted, negatively
charged electrons.
The diameter of a nucleus ranges from about 1.6 – 15 femtometers ( fm )
a.k.a. ‘Fermi’ in
honor of nuclear physicist Enrico Fermi.
1 f m (or Fermi) = 1 quadrillionth (10 -15 ) of a meter
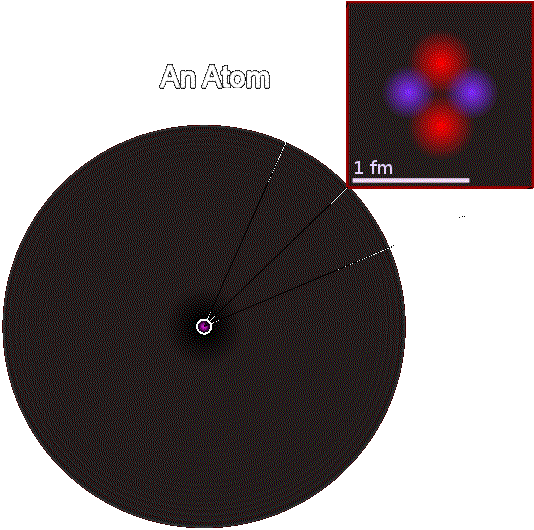
An Atomic Nucleus surrounded by an Electron Cloud
Why Don't
the Negative Electrons
Stick to the Positive Nucleus?
Well, in 1924, Louis de Broglie, a French physics graduate student, delivered
a thesis to Paris University containing his important findings attributing wavelengths to electrons.
It was already known that light waves could behave as particles, called photons, and de Broglie reasoned that nature was symmetric.
Why shouldn't particles behave as waves?
In 1929, after the wave nature of electrons
was demonstrated experimentally, the Nobel Prize for Physics was
conferred on de Broglie for his discovery.
The “de Broglie”
wavelength of a particle is inversely proportional to its momentum :
|
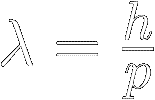
|
[1]
|
|
Where:
λ (lambda) = the particle's wavelength in meters
p = the particle's momentum (mass × velocity) in kg⋅m ⁄ s
ℎ = Planck's constant ≡ 6.626 070 15 × 10 -34 kg⋅m2 ⁄ s
Using equation [1], we can calculate that an electron traveling at a reasonable speed has a wavelength of about
0.01 nm, (a nanometer is one
billionth of a meter).
This length is many thousands of times longer than the
nuclear diameter of 1.6 to 15 f m
(a femtometer is one quadrillionth of a meter).
So, the electron is much more extended in space than a
nucleus. It can't even exist closer to a nucleus
than a couple of electron wavelengths, much less stick to one.
★ Equation [1] also converts a light's wavelength into its photon momentum.
But, since a photon has no rest mass, its momentum is
solely due to the energy of its frequency (f) :
p = h / λ = h × f / c
[ c = the speed of light = λ × f ]
De Broglie wavelengths are usually expressed in nanometer (nm) or Angstrom
(Å) subunits :
1 nm = 10 -9 m
1 Å = 10 -10 m
The Table of Elements
The lightest atom, hydrogen, contains one proton and one electron but weightier atoms
can contain over a hundred of each. Each atomic weight is one element
in the periodic table of chemical elements.
Atoms, themselves, can bond electrochemically with other atoms, sharing electrons to form
larger molecules, compounds and other materials.

In the end, tennis balls bounce, buildings stand up, and aspirin thins the blood,
all because of interacting electromagnetic fields.
|
|
Current — Charge in Motion
|
|
It turns out that the metal atoms
in the periodic table
can bond with each other to form a structure in which many of the atoms' outer electrons
are delocalized—that is,
they're not tied to any particular atomic nucleus or chemical bond.
Metallic structures resemble a 3-D lattice of positive ions
( atoms lacking an electron ) sitting in a ‘ sea ’
of free electrons that can flow en masse through the structure like water through a sieve.
METTALLIC LATTICE
Positive Ions & Delocalized Electrons
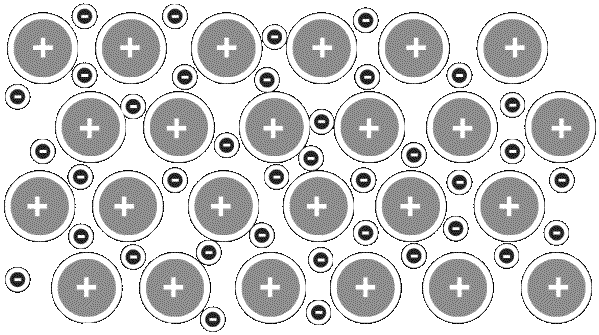
Metals that support such electron flow are called conductors.
They include silver, gold, copper, tin, nickel, zinc and aluminum.
Metallic alloys like brass, bronze and aluminum bronze, are also
conductors.
Materials that don't support electron flow are called insulators.
They include wood, rubber, ceramics, plastic, and glass.
The rate of electron flow is called electric current,
symbol ‘ I ’
from the French phrase “ intensité de courant ”.
Current is the amount of charge
(q) passing a certain point per second (t):
|
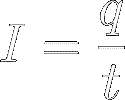
|
[2]
|
|
DC / AC
Direct current (DC) is a one-way
flow of electrons. The intensity of DC may fluctuate but its
direction doesn't.
Alternating current (AC)
is an electron flow that alternates in direction—the electrons
surge forward and back around a center position.
These vibrating electrons create an electric wave of
charged particles that propagates through electrical conductors and components.
In the same way, a vibrating speaker cone creates an
acoustic wave of air particles that propagates through the air.
Click the link to watch an animation of such longitudinal
waves.
Because AC constantly increases, decreases and
changes direction, an average,
root-mean-square
(RMS) value is used to communicate an effective, steady value.
Alternating currents, voltages and power are commonly expressed using RMS values.
Units of Current and Charge
The unit of electric current is
the ampere ( or ‘ amp ’ ),
abbreviated capital A in honor of French mathematician and physicist
André-Marie Ampère (1775-1836), who is considered to be
the father of electrodynamics.
In the International System of Units ( SI ), the ampere is the base unit
of electricity.
◼ There are 7 base units
in the SI: length (meter), time (second), mass (kilogram),
electric current (ampere), temperature (kelvin), amount of substance
(mole), and luminous intensity (candela). All other units
in the SI can be
expressed in terms of these 7.
In the SI, one ampere of current is defined to be a flow of 6.241
509 074 × 1018 elementary
charges ( e ) per second. As stated earlier, that's the number of elementary
charges in one coulomb.
In the SI, electric charge ( Q ) is a unit
that derives from two base units, current ( I ) and time
( t ):
|

|
[3]
|
|
Equation [3] is a rearrangement of equation [2].
So, the SI defines one coulomb to be the amount
of charge that a one ampere current delivers in one second. So,
1 C ≅ (6.24 × 1018 e) × (1) = 6.24 × 1018 e
For this reason, a coulomb of electric charge is sometimes called an
ampere-second.
|
|
Voltage — Separated Charge
|
|
First of all, voltage is an energy
( symbol E).
▶
The unit of energy is the joule,
abbreviated capital J in honor of James Prescott Joule—a 19th century English physicist,
mathematician and brewer who related heat energy to
mechanical energy, laying the foundation for the law of
energy conservation.
What's more, voltage is a potential
or stored energy that results from an object's position or arrangement.
Stored energy is not in use but is ready for use.
For example, potential energy rises when particles of opposite
charge are separated; also when a rock is raised above
Earth. The rise
in energy results from the energy spent
in separating the charges or lifting the rock.
A voltage, symbol V, is the
difference in electrical potential between two points in an electric
field and is defined as energy per unit of charge (q ):
|
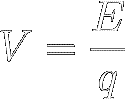
|
[4]
|
|
The SI unit of voltage is the volt,
abbreviated capital ‘ V ’
in honor of Alessandro Volta—an Italian physicist and chemist who
invented the primary battery in 1799.
One volt
is one joule of potential difference per coulomb of charge.
▶
Voltage is sometimes called an electromotive force ( emf )
because it can change an object's motion by transferring energy to it.
Despite its name, however, emf isn't a force. Rather, it's a stored
energy, like that of a stretched rubber band.
|
|
Power
|
|
A transfer of energy ( or " work " ) can be accomplished quickly or slowly,
although doing it quickly takes more power.
For example, it takes more power
to run up a hill than to walk up, even though both ways
will give you the same amount of stored, gravitational potential.
Power (symbol P)
measures an energy transfer per unit of time. In other words,
it's the ‘ speed ’ of energy transfer :
|
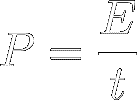
|
[5]
|
|
The unit of power is the watt,
One watt is the amount of power it takes to transfer one joule of energy in one
second.
▶
The watt is abbreviated
‘W’ in honor of James Watt (1736-1819), a
Scottish inventor, mechanical engineer and chemist whose improvements
to steam engine technology drove the Industrial Revolution.
Equation [5] can also be written :
E = P × t
In fact, your electric service is ordinarily billed in units of
kilowatt-hours ( P × t )
instead of joules ( E).
One kilowatt-hour ( kWh ) is the amount
energy transferred by 1,000 watts of power
in 1 hour (3,600 seconds). This amount equals 3.6 megajoules :
1 kW h = 1,000 W x 3,600 s = 3,600,000 J = 3.6 M J

An alkaline AA battery delivers about 9 kJ ( 9,000
joules ) of energy in its
useful life.
An average bolt of lighting delivers 1,000,000 kJ
( 1 billion joules ) of energy in
just 30 microseconds!
Power = Volts × Amps
Take a look at the following algebraic identity, where the two q' s cancel each other out :
|
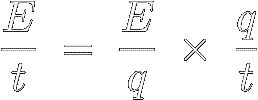
|
[6]
|
|
Also notice that the above three ratios are the definitions of
P, V,
and I ( see equations
[5],
[4], and
[2] ).
So,
this identity shows that the power P of
a current I fueled by a voltage V is :
|

|
[7]
|
|
One watt is the amount of power that drives one amp of current across
a potential difference of one volt.
|
|
Resistance
|
|
In the ordinary world, there are no perfect conductors of electricity. Even in
metals,
electrons collide with ions, losing energy in
the form of heat.
This friction-like impedance to electric current is called resistance.
In electronics, resistance can be used to limit an electric current, or
to establish
a specific voltage. A resistor
is a circuit component that's engineered to provide a precise amount of resistance.
Ohm's Law
Experiments show that the ratio of the voltage V
applied across a
certain
resistor to the current I that gets
pushed through that resistor is a constant.
This ratio is defined to be the "resistance"
(symbol R) of that resistor :
|
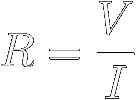
|
[8]
|
|
The unit of resistance is the ohm, abbreviated
Ω (capital Omega because Ohm was a person).
A 1 Ω resistor connected across a potential difference of 1 V will
let a current of 1 A pass through it.
Equation [8] is called Ohm's Law.
When you multiply both sides of
Ohm's Law by I, you find that the
voltage across a resistor is equal to the current through the resistor
multiplied by its resistance :
|
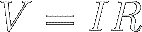
|
[9]
|
|
And when you divide both sides of equation [9] by R,
you find that the current through a resistor is equal to the voltage
across the resistor divided by its resistance :
|
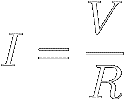
|
[10]
|
|
A trick to help remember the three permutations of Ohm's Law (equations [8], [9] and [10])
is to substitute Vulture,
Rabbit, and Indian
for V, R,
and I.
The Rabbit sees the Vulture over the Indian, the
Vulture sees the Indian next to the Rabbit, and the
Indian sees the Vulture over the Rabbit.
Power Transfer
When electrons flow through a resistor, the resistor must be able to dissipate
(move away) all the heat generated by its internal
electron-ion collisions.
Otherwise, the resistor will overheat and burn out.
Therefore, every resistor has a power rating specifying the maximum
power that the resistor can safely dissipate.
Ohm's Law reveals how much power a certain resistance R
must dissipate
when a current I flows through it .
In equation [7] ( P = V × I ) ,
just replace V with
IR :
|
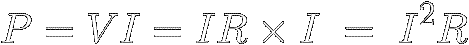
|
[11]
|
|
The Law also reveals how much power a certain resistance R
must dissipate when a voltage V is applied across it.
In equation [7], just replace I
with V/R :
|
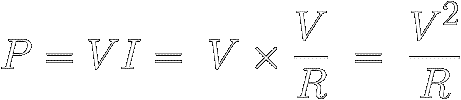
|
[12]
|
|
Resistor Construction
Like garden hoses, wires that are long and thin offer more
resistance to current than do ones that are short and fat.
|

One way to manufacture a resistor is to simply coil up a long, thin piece of wire.
Such wirewound (a.k.a.
sandblock or cement)
resistors can be very precise and handle large currents.
|
|
Other resistors are made from materials like carbon
that fall in between a conductor and
an insulator. These materials have relatively few
delocalized electrons.

These vintage, carbon composition resistors are composed of tiny carbon particles bound with clay.
|
|
Many modern resistors are made from laser-cut, helical tracks of carbon
or metal film.
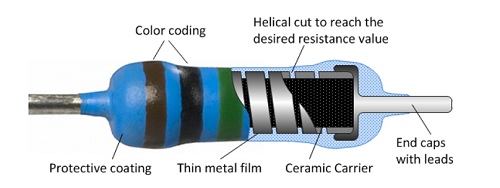
|

|
|